| Double electron capture is a decay mode of atomic nucleus. For a nuclide (A, Z) with number of nucleons A and atomic number Z, double electron capture has to occur when the mass of the nuclide of (A, Z-2) is lower.
|
| In this mode of decay, two of the orbital electrons are captured by two protons in the nucleus, forming two neutrons. Two neutrinos are emitted in the process. Since the protons are changed to neutrons, the number of neutrons increases by 2, the number of protons Z decreases by 2, and the atomic mass A remains unchanged. By changing the number of protons, double electron capture transforms the nuclide into a new element.
|
| Example: |
|
|
| There exist 35 naturally occurring isotopes that can undergo double electron capture. However, there are no confirmed observations of this process. The one reason is that the probability of double electron
capture is enormously small (the theoretical predictions of half-lives for this mode lies well above 1020 years). The second reason is that the only detectable particles created in this process
are X-rays and Auger electrons that are emitted by the excited atomic shell. In the range of their energies (~1 to 10 keV), the background is usually high. Thus, the experimental detection of double electron capture is more difficult than that for double beta decay.
|
| If the mass difference between the mother and daughter atoms is more than two masses of electron (1.022 MeV), the energy released in the process is enough to allow another mode of decay: electron capture with positron emission. It occurs simultaneously with double electron capture, their branching ratio depending on nuclear properties. When
the mass difference is more than four electron masses (2.044 MeV), the third mode - double positron decay - is allowed. Only 6 naturally occurring nuclides can decay via these three modes simultaneously.
|
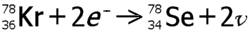
 See Also
See Also