| Electron Capture |
| Electron capture is a decay mode for isotopes that will occur when there are too many protons in the nucleus of an atom, and there isn't enough energy to emit a positron; however, it continues to be a viable decay mode for radioactive isotopes that can decay by positron emission. If the energy difference between the parent atom and the daughter one is less than 1.022 MeV, positron emission is forbidden and electron capture is the sole decay mode. For example, Rubidium-83 will decay to Krypton-83 solely by electron capture (the energy difference is about 0.9 MeV). |
| In this case, one of the orbital electrons, usually from the K or L electron shell (K-electron capture, also K-capture, or L-electron capture, L-capture), is captured by a proton in the nucleus, forming a neutron and a neutrino. Since the proton is changed to a neutron, the number of neutrons increases by 1, the number of protons decreases by 1, and the atomic mass number remains unchanged. By changing the number of protons, electron capture transforms the nuclide into a new element. The atom ends up in excited state, with a missing electron in inner shell. The atom in its excited state will emit X-rays (a type of electromagnetic radiation) and/or Auger electrons. The electron in an outer shell falls into the inner shell releasing energy, emitted as the x-ray. Because of this electron capture is most likely to happen in larger neculides. |
| examples: |
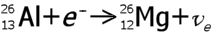 |
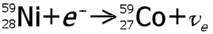 |
| It is of note that radioactive isotopes which go by pure electron capture can, in theory, be inhibited from radioactive decay if they are fully ionized ("stripped" is sometimes used to describe such ions). It is hypothesized that such elements, if formed by the r-process in exploding supernovae, are ejected fully ionized and so do not undergo radioactive decay as long as they do not encounter electrons in outer space. Anomalies in elemental distributions are thought to be partly a result of this effect on electron capture. |
 See Also See Also
|
| Double Electron Capture and Electron Emission. |