| Reactions of Rubidium |
 Reactions with water Reactions with water |
| Rubidium metal reacts very rapidly with water to form a colourless solution of rubidium hydroxide (RbOH) and hydrogen gas (H2). The reaction is very exothermic. The resulting solution is basic because of the dissolved hydroxide. The reaction is so fast that if the reaction is carried
out in a glass vessel, the glass container may well shatter. The reaction is slower than that of caesium (below rubidium in the periodic table), but faster than that of potassium (above rubidium in the periodic table).
|
2Rb(s) + 2H2O2 2RbOH(aq) + H2(g) 2RbOH(aq) + H2(g) |
 Reactions with air Reactions with air |
| Rubidium is very soft and easily cut, the resulting surface is bright and shiny. However, this surface soon tarnishes because
of reaction with oxygen and moisture from the air. If rubidium is burned in air, the result is mainly formation of dark brown rubidium superoxide,
RbO2.
|
Rb(s) + O2(g) RbO2(s) RbO2(s) |
 Reactions with halogens Reactions with halogens |
| Rubidium metal reacts vigorously with all the halogens to form rubidium(I) halides. |
2Rb(s) + F2(g) 2RbF(s) 2RbF(s) |
2Rb(s) + Cl2(g) 2RbCl(s) 2RbCl(s) |
2Rb(s) + Br2(g) 2RbBr(s) 2RbBr(s) |
2Rb(s) + I2(g) 2RbI(s) 2RbI(s) |
 Reactions with acids Reactions with acids |
| Rubidium metal dissolves readily in dilute sulphuric acid (H2SO4) to form solutions containing the Rb(I) ion together with hydrogen gas, H2.
|
2Rb(s) + H2SO4 2Rb+(aq) + SO42-(aq) + H2(g) 2Rb+(aq) + SO42-(aq) + H2(g) |
 Reactions with bases Reactions with bases |
| Rubidium metal reacts very rapidly with water to form a colourless basic solution of rubidium hydroxide (RbOH) and hydrogen gas (H2). The reaction continues even when the solution becomes basic. The resulting solution is basic because of the dissolved hydroxide.
As the reaction proceeds, the concentration of base increases. The reaction is very exothermic. The reaction is so fast that if the reaction is carried out in a glass vessel, the glass container may well shatter! The
reaction is slower than that of caesium (below rubidium in the periodic table), but faster than that of potassium (above rubidium in the periodic table).
|
2Rb(s) + 2H2O 2RbOH(aq) + H2(g) 2RbOH(aq) + H2(g) |
| |
 Reactions with water
Reactions with water 2RbOH(aq) + H2(g)
2RbOH(aq) + H2(g) Reactions with air
Reactions with air RbO2(s)
RbO2(s) Reactions with halogens
Reactions with halogens 2RbF(s)
2RbF(s) 2RbCl(s)
2RbCl(s) 2RbBr(s)
2RbBr(s) 2RbI(s)
2RbI(s) Reactions with acids
Reactions with acids 2Rb+(aq) + SO42-(aq) + H2(g)
2Rb+(aq) + SO42-(aq) + H2(g) Reactions with bases
Reactions with bases 2RbOH(aq) + H2(g)
2RbOH(aq) + H2(g) Reactions with water
Reactions with water 2RbOH(aq) + H2(g)
2RbOH(aq) + H2(g) Reactions with air
Reactions with air RbO2(s)
RbO2(s) Reactions with halogens
Reactions with halogens 2RbF(s)
2RbF(s) 2RbCl(s)
2RbCl(s) 2RbBr(s)
2RbBr(s) 2RbI(s)
2RbI(s) Reactions with acids
Reactions with acids 2Rb+(aq) + SO42-(aq) + H2(g)
2Rb+(aq) + SO42-(aq) + H2(g) Reactions with bases
Reactions with bases 2RbOH(aq) + H2(g)
2RbOH(aq) + H2(g)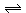 Rb(s)
Rb(s)