 Discovery Information Discovery Information
|
| Who: Fredrich Soddy, John Cranston, Otto Hahn, Lise Meitner |
| When: 1917 |
| Where: England/France |
|
 Name Origin Name Origin
|
| protos (first); its is the parent of actinium, which is formed by radioactive decay.
|
| "Protactinium" in different languages. |
|
 Sources Sources
|
| Does not occur in nature. Found among fission products of uranium, thorium, and plutonium.
|
| Protactinium occurs in pitchblende to the extent of about 1 part 231Pa to 10 million of ore. Some ores from the Democratic Republic of the Congo have about 3 ppm.
|
|
 Abundance Abundance
|
| Earth's Crust: 1 x 10-8 ppm
|
| Seawater: 2 x 10-11 ppm
|
|
 Uses Uses
|
| Because of its scarcity, high radioactivity and toxicity, there are currently no uses for protactinium outside of basic scientific
research.
|
|
 History History
|
| An element between thorium and uranium was predicted to exist by Mendeleev in 1871. In 1900 William Crookes isolated protactinium as a radioactive material from uranium which he could not identify
|
| Protactinium was first identified in 1913, when Kasimir Fajans and O. H. Gohring encountered short-lived isotope 234m-Pa, with a half-life of about 1.17 minutes, during their studies of the decay chain of 238-U. They gave the new element the name Brevium (Latin
brevis, brief, short); the name was changed to Protoactinium in 1918 when two groups of scientists (Otto Hahn and Lise Meitner of Germany and Frederick Soddy and John Cranston of the UK) independently discovered 231-Pa, and shortened to Protactinium in 1949.
|
| Aristid V. Grosse prepared 2 mg of Pa2O5 in 1927, and later on managed to isolate Protactinium for the first time in 1934 from 0.1 mg of Pa2O5, first converting the oxide to an iodide and then cracking it in a high vacuum by an electrically heated filament.
|
| In 1961, the United Kingdom Atomic Energy Authority was able to produce 125 g of 99.9% pure protactinium, processing 60 tons of waste material in a 12-stage
process and spending 500,000 USD; this was the world's only supply of the element for many years to come, and it is reported
that the metal was sold to laboratories for a cost of 2,800 USD / g in the following years.
|
|
 Notes Notes
|
| It is superconductive at temperatures below 1.4 K (-271.74°C). |
| 29 radioisotopes of protactinium have been characterized, with the most stable being 231Pa with a half life of 32760 years, 233Pa with a half-life of 26.967 days, and 230Pa with a half-life of 17.4 days. All of the remaining radioactive isotopes have half-lives that are less than 1.6 days, and the majority of these have half lifes that are less than 1.8 seconds.
|
|
 Hazards Hazards
|
| Protactinium is both toxic and highly radioactive. |

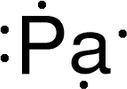

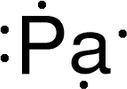
 Discovery Information
Discovery Information
 Name Origin
Name Origin
 Sources
Sources
 Abundance
Abundance
 Uses
Uses
 History
History
 Notes
Notes
 Hazards
Hazards