 Discovery Information Discovery Information
|
| Who: Marguerite Perey |
| When: 1939 |
| Where: France |
|
 Name Origin Name Origin
|
| From France. |
| "Francium" in different languages. |
|
 Sources Sources
|
| Formed by decay of actinium. Chemical properties similar to caesium. Decays to radium or astatine.
|
| A small number of pictures of francium have been taken, but only of at the most 350,000 atoms at a time. |
|
|
 Uses Uses
|
| Francium has no known uses. |
|
 History History
|
| As early as 1870, it was thought by chemists that there should be an alkali metal beyond caesium, with an atomic number of 87. It was then referred to by the provisional name eka-caesium. Research teams attempted to locate
and isolate this missing element, and at least four false claims were made that the element had been found before an authentic
discovery was made.
|
| Eka-caesium was truly discovered in 1939 by Marguerite Perey of the Curie Institute in Paris, France, when she purified a sample of actinium-227 which had been reported to have a decay energy of 220 keV. However, Perey noticed decay particles with an energy level
below 80 keV. Perey thought this decay activity might have been caused by a previously unidentified decay product, one which
was separated during purification, but emerged again out of the pure actinium-227. Various tests eliminated the possibility
of thorium, radium, lead, bismuth, or thallium being the unknown element. The new product exhibited chemical properties of an alkali metal (such as co-precipitating with
caesium salts), which led Perey to believe that it was element 87, caused by the alpha decay of actinium-227. Perey then attempted
to determine the proportion of beta decay to alpha decay in actinium-227. Her first test put the alpha branching at 0.6%,
which she later revised to 1%.
|
| Perey named the new isotope actinium-K, which we now refer to as francium-223, and in 1946, she proposed the name catium for her newly-discovered element,
as she believed it to be the most electropositive cation of the elements. Irene Joliot-Curie, one of Perey's supervisors,
opposed the name due to its connotation of cat rather than cation. Perey then suggested francium as an homage to the country
in which she discovered it. This name was officially adopted by the International Union of Chemists in 1949. and assigned
the symbol Fa; but, this abbreviation was revised to the current Fr shortly thereafter. Francium was the last naturally occurring
element to be discovered, following rhenium in 1925. Further research into francium's structure was carried out by Sylvain
Lieberman and his team at CERN in the 1970s and 1980s, among others.
|
|
 Notes Notes
|
| The longest lived isotope, 223Fr, a daughter of 227Ac, has a half-life of 22 minutes. This is the only isotope of francium occurring in nature, but at most there is only 20-30 g of the element present in the earth's crust at any one
time. No weighable quantity of the element has been prepared or isolated. There are 34 known isotopes.
|
| It is notable for having the lowest electronegativity and electron affinity of all the elements.
|
| Originally names Actinium K. |
|
 Hazards Hazards
|
| Francium is radioactive. |

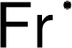

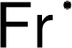
 Discovery Information
Discovery Information
 Name Origin
Name Origin
 Sources
Sources
 Uses
Uses
 History
History
 Notes
Notes
 Hazards
Hazards