 Discovery Information Discovery Information
|
| Who: Albertus Magnus |
| When: 1250 |
| Where: Unknown |
|
 Name Origin Name Origin
|
| Greek: arsenikos (male); Latin: arsenicum. |
| "Arsenic" in different languages. |
|
 Sources Sources
|
| Arsenopyrite, also unofficially called mispickel, (FeAsS) is the most common arsenic-bearing mineral. On roasting in air,
the arsenic sublimes as arsenic (III) oxide leaving iron oxides.
|
|
 Abundance Abundance
|
| Universe: 0.008 ppm (by weight) |
| Carbonaceous meteorite: 1.8 ppm |
| Earth's Crust: 1.5 ppm |
| Seawater: |
| Atlantic surface: 1.45 x 10-3 ppm
|
| Atlantic deep: 1.53 x 10-3 ppm
|
| Pacific surface: 1.45 x 10-3 ppm
|
| Pacific deep: 1.75 x 10-3 ppm
|
| Human: |
| 50 ppb by weight |
| 4 ppb by atoms |
|
 Uses Uses
|
| Used as a deadly poison (in various agricultural insectisides and poisions), in shotgun pellets, metal for mirrors, glass,
lasers. Gallium Arsenide is an important semiconductor used in integrated circuits and light-emitting diodes (LEDs).
|
|
 History History
|
| Arsenic has been known and used in Persia and elsewhere since ancient times. As the symptoms of arsenic poisoning were somewhat
ill-defined, it was frequently used for murder until the advent of the Marsh test, a sensitive chemical test for its presence.
|
| Due to its use by the ruling class to murder one another and its potency and discreetness, arsenic has been called the Poison
of Kings and the King of Poisons.
|
| During the Bronze Age, arsenic was often included in bronze, which made the alloy harder (so-called "arsenical bronze"). |
| Albertus Magnus (Albert the Great, 1193-1280) is believed to have been the first to isolate the element in 1250. In 1649 Johann Schroder published two ways of preparing arsenic.
|
|
 Notes Notes
|
| In the Victorian era, 'arsenic' (colourless, crystalline, soluble 'white arsenic') was mixed with vinegar and chalk and eaten
by women to improve the complexion of their faces, making their skin paler to show they did not work in the fields. Arsenic
was also rubbed into the faces and arms of women to 'improve their complexion'. The accidental use of arsenic in the adulteration
of foodstuffs led to the Bradford sweet poisoning in 1858, which resulted in approximately 20 deaths and 200 people taken
ill with arsenic poisoning.
|
|
 Hazards Hazards
|
| Very toxic. Arsenic is a carcinogen, associated with lung cancer when inhaled. Contact with skin can result in skin cancer. Also damage to intestines and liver.
Toxic when ingested. May cause reproductive disorders. It is found in pesticides and wood preservatives.
|
| Harmful in the environment, very toxic to aquatic organisms. May cause long term damage. |

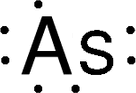

 Discovery Information
Discovery Information
 Name Origin
Name Origin
 Sources
Sources
 Abundance
Abundance
 Uses
Uses
 History
History
 Notes
Notes
 Hazards
Hazards