 Discovery Information Discovery Information
|
| Who: A. Ghiorso, A: Los Alamos, U of California
|
| When: 1953 |
| Where: United States |
|
 Name Origin Name Origin
|
| After the scientist Enrico Fermi. |
| "Fermium" in different languages. |
|
 Sources Sources
|
| Completely synthetic element. Produced by bombarding lighter transuranium elements with still lighter particles or by neutron capture. 253Fm can be produced in nanogram (10-9) quantities by bombarding 239Pu with neutrons.
|
|
|
 Uses Uses
|
| None. |
|
 History History
|
| Fermium (after Enrico Fermi) was first discovered by a team led by Albert Ghiorso in 1952. The team found 255Fm in the debris of the first hydrogen bomb explosion (see Operation Ivy). That isotope was created when 238U combined with 17 neutrons in the intense temperature and pressure of the explosion (eight beta decays also occurred to create the element). The work
was overseen by the University of California Radiation Laboratory, Argonne National Laboratory, and Los Alamos Scientific
Laboratory whose team members included Ghiorso, Stanley G. Thompson, Gary H. Higgins, Glenn T. Seaborg (from the Radiation Laboratory and Department of Chemistry of the
University of California), Martin H. Studier, P.R. Fields, Sherman M. Fried, H. Diamond, J.F. Mech, G.L. Pyle, John R. Huizenga,
A. Hirsch, W.M. Manning (from the Argonne National Laboratory), C.I. Browne, H. Louise Smith, and R.W. Spence (from the Los
Alamos Scientific Laboratory). Samples of sea coral impacted from the first thermonuclear explosion of November 1952 were
used.
|
| All these findings were kept secret until 1955 due to Cold War tensions, however. In late 1953 and early 1954 a team from
the Nobel Institute of Physics in Stockholm bombarded a 238U target with 16O ions, producing an alpha-emitter with an atomic weight of ~250 and with 100 protons (in other words, element 250100). The Nobel team did not claim discovery but the isotope they produced was later positively identified as 250Fm.
|
|
 Notes Notes
|
| Fermium's chemical properties are largely unknown. Fermium is a radioactive rare earth metal. The longest living isotope is 257Fm with a half-life of 80 days. It is of no commercial importance.
|
|

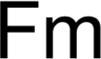

 Discovery Information
Discovery Information
 Name Origin
Name Origin
 Sources
Sources
 Uses
Uses
 History
History
 Notes
Notes